KN-671


2016 1,2
KEYNOTE-010
PD-L1 ≥1% – 2L MONOTHERAPY
KEYTRUDA® as monotherapy is indicated for the treatment of metastatic non-small cell lung carcinoma in adults whose tumours express PDL1 with a ≥ 1% TPS and who have received at least one prior chemotherapy regimen.
Patients with EGFR or ALK positive tumour mutations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA.
2018 1,3
KEYNOTE-189
NSQ – 1L COMBINATION THERAPY
KEYTRUDA®, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of metastatic nonsquamous non-small cell lung carcinoma in adults whose tumours have no EGFR or ALK positive mutations.
2019 1,4
KEYNOTE-042
PD-L1 ≥ 1%– 1L MONOTHERAPY
KEYTRUDA® as monotherapy is indicated for the first-line treatment
of stage III where patients are not candidates for surgical resection or definitivechemoradiation or metastatic non-small cell lung carcinoma in adults whose tumours express PD-L1 with a ≥ 1% tumour proportion score (TPS) with no EGFR or ALK positive tumour mutations.
2019 1,5
KEYNOTE-407
SQ – 1L COMBINATION THERAPY
KEYTRUDA®, in combination with carboplatin and either paclitaxel or nab-paclitaxel, is indicated for the first-line treatment of metastatic squamous non-small cell lung carcinoma in adults.
2023 1,6
KEYNOTE-091
ADJUVANT MONOTHERAPY
KEYTRUDA® as monotherapy is indicated as adjuvant treatment following resection and platinum-based chemotherapy for adult patients with Stage IB (T2a ≥4 cm), II, or IIIA non-small cell lung carcinoma.
2024 1,7
KEYNOTE-671 NEOADJUVANT COMBINATION TREATMENT, THEN CONTINUED AS ADJUVANT MONOTHERAPY
KEYTRUDA®,For the treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC in combination with platinum-containing chemotherapy as neoadjuvant treatment, and then continued as monotherapy as adjuvant treatment.
1L: first line; 2L: second line; ALK: anaplastic lymphoma kinase; EGFR: epidermal growth factor receptor; nab-paclitaxel: paclitaxel
protein-bound; NSCLC: non-small cell lung cancer; NSQ: nonsquamous; PD-L1: programmed death ligand 1; SQ: squamous; TPS: tumor proportion score.
KEYTRUDA®, is used for the treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC in combination with platinum- containing chemotherapy as neoadjuvant treatment, and then continued as a single agent as adjuvant treatment after surgery.1
References:
- KEYTRUDA® Prescribing Information.
- Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, Zhao H, Yu J, Paciga M, Goldberg KB, McKee AE, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist. 2017 Nov;22(11):1392-1399. doi: 10.1634/theoncologist.2017-0078. Epub 2017 Aug 23. PMID: 28835513; PMCID: PMC5679831
- Research C for DE and R. FDA grants regular approval for pembrolizumab in combination with chemotherapy for first-line treatment of metastatic non-squamous NSCLC. FDA. 2018 Nov 3; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval pembrolizumab combination- chemotherapy-first-line-treatment-metastatic. Accessed Oct. 22, 2024
- Research C for DE and R. FDA expands pembrolizumab indication for first-line treatment of NSCLC (TPS≥1). FDA. Published online December 20, 2019. https://www.fda.gov/drugs/fda-expands-pembrolizumab-indicationfirstlinetreatment-nsclc-tps-1 Accessed November 2, 2024.
- Research C for DE and R. FDA approves pembrolizumab in combination with chemotherapy for first-line treatment of metastatic squamous NSCLC. FDA. 2019 Available from: https://www.fda.gov/drugs/fda-approves-pembrolizumab-combination-chemotherapy-first-line-treatment-metastatic-squamous-nsclc. Accessed Oct. 22, 2024
- Research C for DE and R. FDA approves pembrolizumab as adjuvant treatment for non-small cell lung cancer. FDA. 2023 Jan 26; Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-non-small-cell-lung-cancer. Accessed Oct. 22, 2024
- Research C for DE and R. FDA approves neoadjuvant/ adjuvant pembrolizumab for resectable non-small cell lung cancer. FDA. 2023; Available from: https://www.fda. gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-adjuvant-pembrolizumab-resectable-non-small-cell-lung-cancer. Accessed Oct. 22, 2024

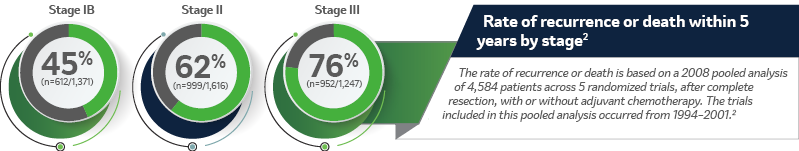
Lung Adjuvant Cisplatin Evaluation: A Pooled Analysis by the LACE Collaborative Group2
The Lung Adjuvant Cisplatin Evaluation (LACE) study was a pooled analysis of 5 randomized trials conducted by the LACE Collaborative Group. The study evaluated the use of cisplatin based chemotherapy as an adjuvant treatment for patients with NSCLC. The primary endpoint was overall survival (OS) and a secondary endpoint was disease-free survival (DFS).2
Study population2
Individual patient data were collected and pooled from 5 trials, including 4,584 patients who underwent complete resection. Of these patients, 2,281 received adjuvant chemotherapy. The interactions between patient subgroups, or treatment types, and chemotherapy effect on OS were analyzed using hazard ratios and log-rank tests stratified by trial.
Inclusion and exclusion criteria2
Trials eligible for inclusion were those that either randomly assigned more than 300 patients with completely resected NSCLC to receive postoperative cisplatin-based chemotherapy versus no chemotherapy or cisplatin-based chemotherapy plus postoperative radiotherapy (administered sequentially) versus postoperative radiotherapy alone.
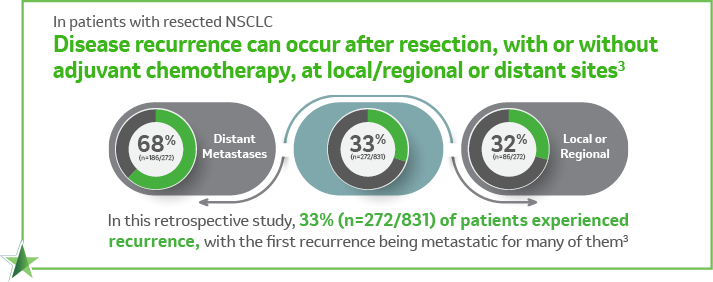
Adjuvant treatment patterns and outcomes in patients with stage IB-IIIA non-small cell lung cancer in France, Germany, and the United Kingdom based on the LuCaBIS burden of illness study3
A retrospective observational study consisting of data collected via medical record abstraction among 831 patients with complete resection of stage IB–IIIA NSCLC per AJCC 7th edition in France, Germany, and the United Kingdom. Eligible patients were diagnosed between January 1, 2009, and December 31, 2011, with a median follow-up time of 26 months.3
Nearly half of the patients (48.4%; n=402/831) received adjuvant systemic therapy. The primary objective of the study was to identify and quantify the treatment patterns of these patients. As a second objective, the study also aimed to evaluate disease recurrence and progression for the same patients.3
References:
- KEYTRUDA® Prescribing Information.
- Pignon JP, Tribodet H, Scagliotti LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008 Jul 20;26(21):3552-9. doi: 10.1200/JCO.2007.13.9030.
- Chouaid C, Danson S, Andreas S, Adjuvant treatment patterns and outcomes in patients with stage IB-IIIA non-small cell lung cancer in France, Germany, and the United Kingdom based on the LuCaBIS burden of illness study. Lung Cancer. 2018 Oct;124:310-316. doi:10.1016/j.lungcan.2018.07.042
KEYNOTE-671 Study Design1,2
A randomized, double-blind, multicenter, placebo-controlled, phase 3 study in patients with resectable stage II, IIIA, or IIIB (N2) NSCLC per AJCC 8th edition.2
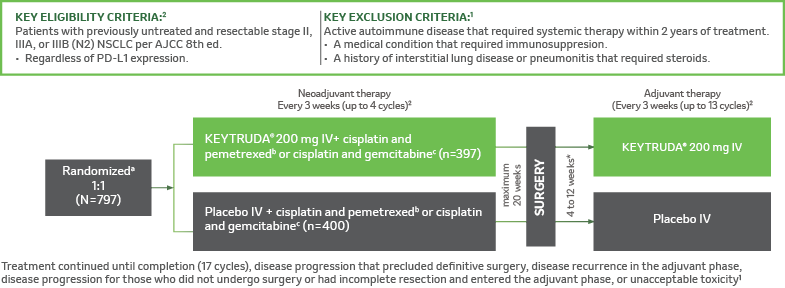

aRandomization was stratified by stage (II vs III), tumor PD-L1 expression (TPS <50% ≥50%), histology (squamous vs nonsquamous), and geographic region (East Asia vs non-East Asia)2, bCisplatin 75 mg/m2 IV Q3W + pemetrexed 500 mg/m2 IV on day 1 of each 21-day cycle2, CCisplatin 75 mg/m2 IV Q3W + gemcitabine 1,000 mg/m2 IV on days 1 and 8 of each 21-day cycle, dAssessed by blinded independent pathology review3, AJCC = American Joint Committee on Cancer; ECOG PS = Eastern Cooperative Oncology Group performance status; IV = intravenous; NSCLC = non-small cell lung carcinoma; PD-L1 = programmed death ligand 1; Q3W = every 3 weeks; RECIST v1.1 = Response Evaluation Criteria in Solid Tumors v1.1; TPS = tumor proportion score.
*In some participants radiotherapy were continued until the maximum number of administrations was reached or until the occurrence of disease progression or recurrence, the occurrence of unacceptable toxic effects, a decision by the investigator to stop administration, withdrawal of consent, or other reasons.2
References:
- KEYTRUDA® Prescribing Information.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.
- Protocol for Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

Kaplan-Meier Estimates of OS in KEYNOTE-6712,a
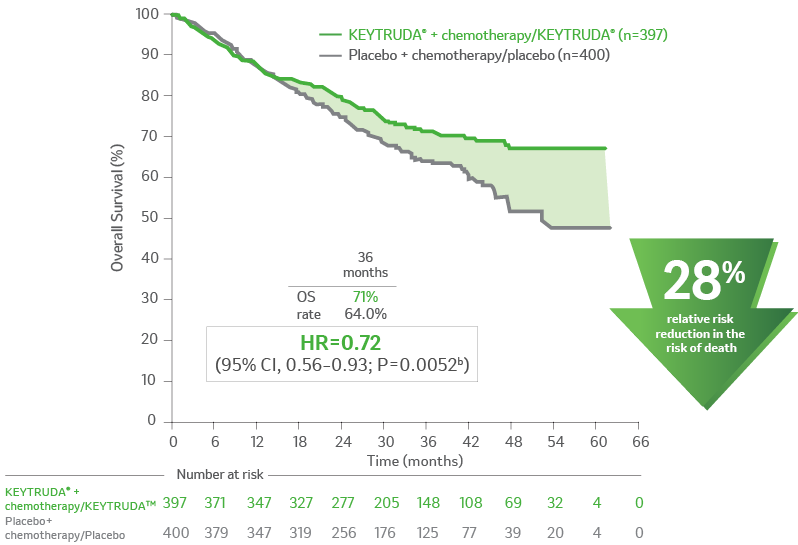

aOS defined as time from randomization to death from any cause.2
bthe multiplicity-adjusted superiority threshold for overall survival at the second interim analysis was one-sided p=0.0054.
CI = confidence interval; HR = hazard ratio; NR = not reached; NSCLC = non–small cell lung carcinoma; OS = overall survival; PD-L1 =
programmed death ligand 1.
References:
- KEYTRUDA® Prescribing Information.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, RodrÃguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

Kaplan-Meier Estimates of EFS in KEYNOTE-6712
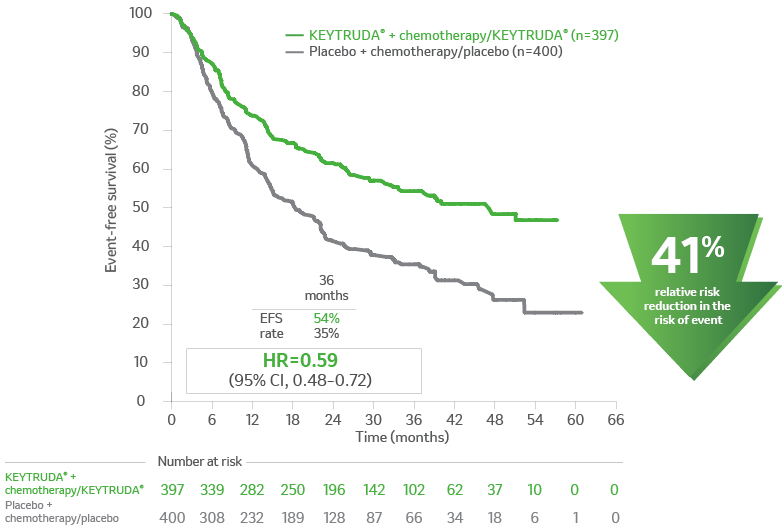
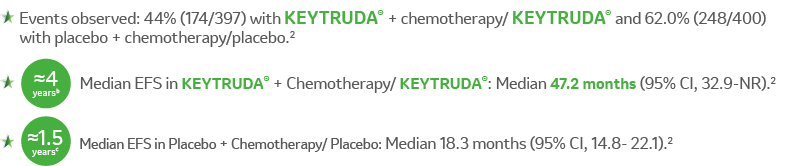
aEFS is defined as the time from randomization to the first occurrence of local progression that precluded the planned surgery, unresectable tumor at the time of surgery, progression or recurrence according to RECIST v1.1, or death from any cause.2
b3.933 years (47.2 months).
c1.525 years (18.3 months).
CI = confidence interval; EFS = event-free survival; HR = hazard ratio; NR = not reached; NSCLC = non–small cell lung
carcinoma; PD-L1 = programmed death ligand 1; RECIST v1.1 = Response Evaluation Criteria In Solid Tumors v1.1.
References:
- KEYTRUDA® Prescribing Information.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

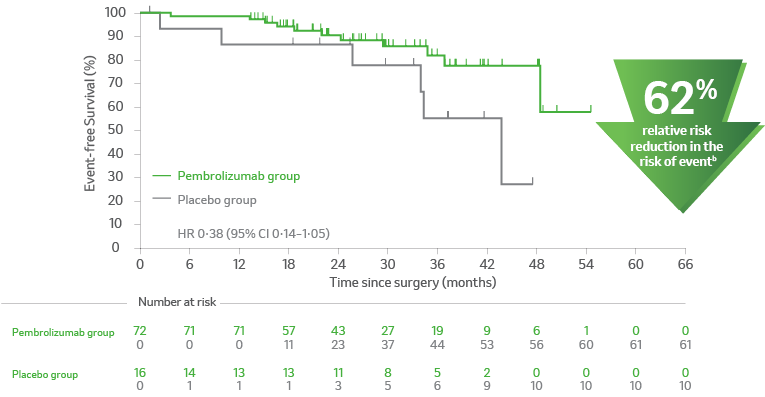
Kaplan-Meier estimates of event-free survival from the time of in-study surgery in subgroups based on pathologic response. This is a post hoc analysis.2
A. Participants with pCR2
B. Participants without pCR2
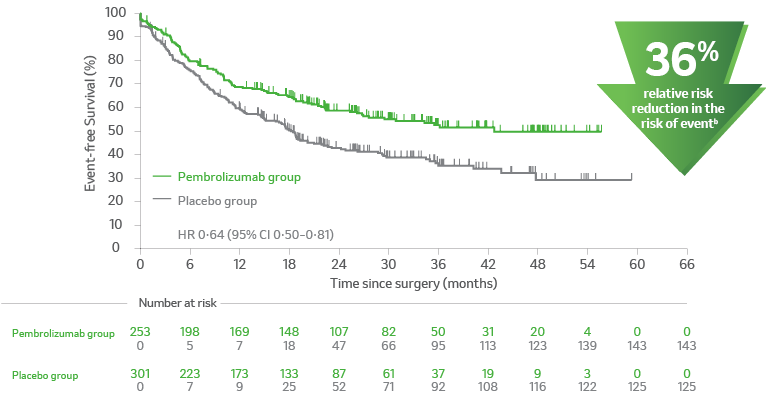
apCR is defined as the absence of residual invasive cancer on hematoxylin and eosin stained slides of the resected lung specimen and lymph nodes following completion of neoadjuvant therapy3, central examination by a pathologist.3
bProgression, recurrence or death.4
CI = confidence interval; EFS = event-free survival; NSCLC = non–small cell lung carcinoma; pCR = pathological complete response; PD-L1 = programmed death ligand 1.
References:
- KEYTRUDA® Prescribing Information.
- Supplementary of Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.
- Protocol for Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

Event-free survival in prespecified and post hoc subgroups (Median Follow-Up Time: 3 years).2
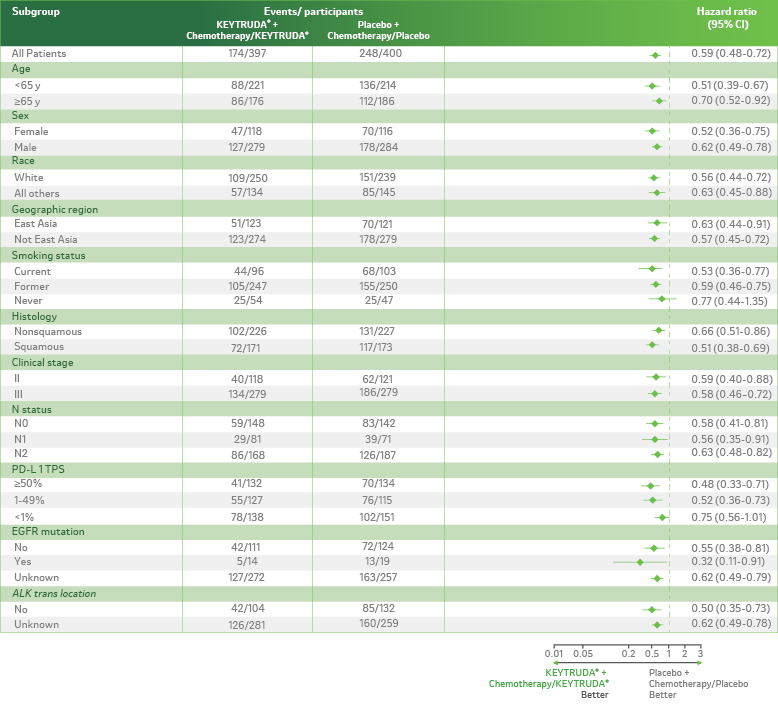
LIMITATION: KEYNOTE-671 was not powered to detect differences in the treatment effect in these subgroups; therefore, results from this exploratory analysis should be interpreted with caution because of the modest patient numbers and potential imbalances in baseline characteristics within the subgroups.
ALK = anaplastic lymphoma kinase; CI = confidence interval; EFS = event-free survival; EGFR = epidermal growth factor receptor; NSCLC = non–small cell lung carcinoma; PD-L1 = programmed death ligand 1; TPS = tumor proportion score.
References:
- KEYTRUDA® Prescribing Information.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

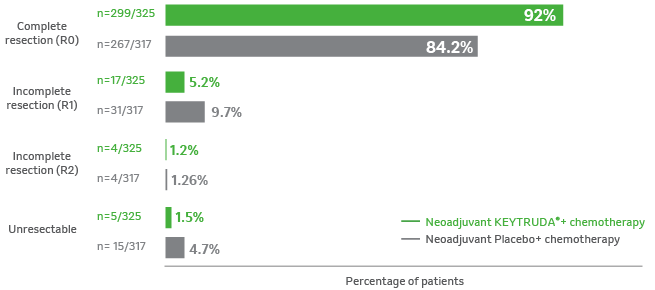
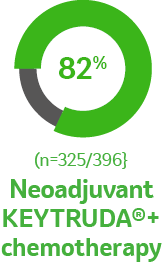
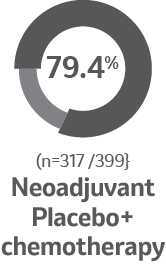
Surgical outcomes for the patients who underwent
in-trial surgery as planned2
Percentage of patients who underwent in-trial surgery2
References:
- KEYTRUDA® Prescribing Information.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

Adverse events occurring in patients with resectable NSCLC receiving KEYTRUDA® in combination with platinum-containing chemotherapy, given as neoadjuvant treatment and continued as monotherapy adjuvant treatment in KEYNOTE-671, were generally similar to those occurring in patients in other clinical trials across tumor types receiving KEYTRUDA® in combination with chemotherapy.1
In the neoadjuvant phase of KEYNOTE-671.2,a
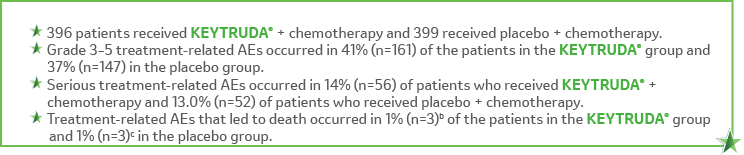
In the adjuvant phase of KEYNOTE-671.2,d

Treatment-related Adverse Reactions in ≥10% of Patients Who Received KEYTRUDA®+ Chemotherapy/KEYTRUDA® and Placebo + Chemotherapy/Placebo3
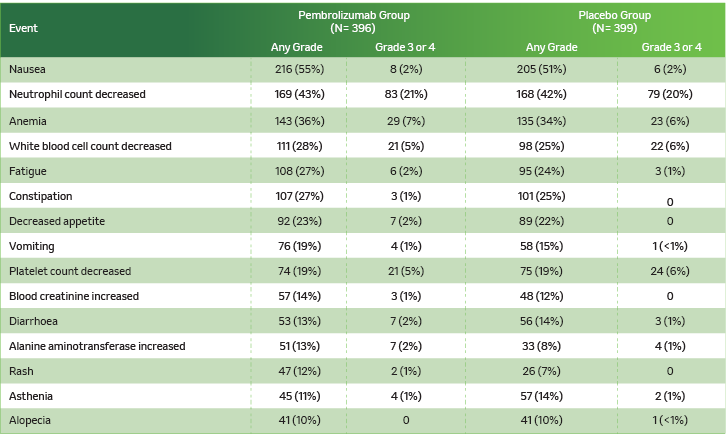
Data are n (%). Treatment-related adverse events were adverse events considered to be related to pembrolizumab or placebo by the investigator.

aThe neoadjuvant/surgery phase began with the first dose of neoadjuvant therapy and ended with the first dose of adjuvant pembrolizumab or placebo. Treatment-related adverse events were adverse events considered to be related to chemotherapy, pembrolizumab, or placebo by the investigator. bOne participant each due to immune-mediated lung disease, pneumonia, and sudden cardiac death.2 cOne participant each due to acute coronary syndrome, pneumonia, and pulmonary hemorrhage.2 dThe adjuvant phase began with the first dose of adjuvant therapy. Treatment-related adverse events were adverse events considered to be related to pembrolizumab or placebo by the investigator.eDue to atrial fibrillation.
AE = adverse event; NSCLC = non-small cell lung carcinoma.
References:
- KEYTRUDA® Prescribing Information.
- Supplementary of Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.

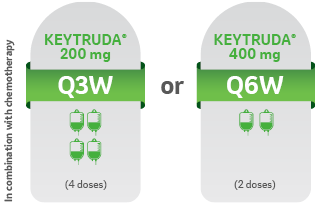
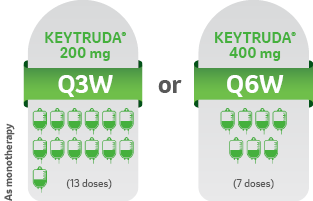
START KEYTRUDA® IN COMBINATION WITH CHEMOTHERAPY, THEN CONTINUE TREATMENT AFTER SURGERY AS MONOTHERAPY KEYTRUDA® can be administered as 200 mg Q3W or 400 mg Q6W1
Administered as an intravenous infusion, after dilution, over 30 minutes1
NEOADJUVANT TREATMENT1
For 12 weeks or until disease progression that precludes definitive surgery or unacceptable toxicity NEOADJUVANT TREATMENT1
ADJUVANT TREATMENT1
For 39 weeks or until disease recurrence or unacceptable toxicity
NSCLC = non-small cell lung carcinoma; Q3W = every 3 weeks; Q6W = every 6 weeks.
Reference:
- KEYTRUDA® Prescribing Information.
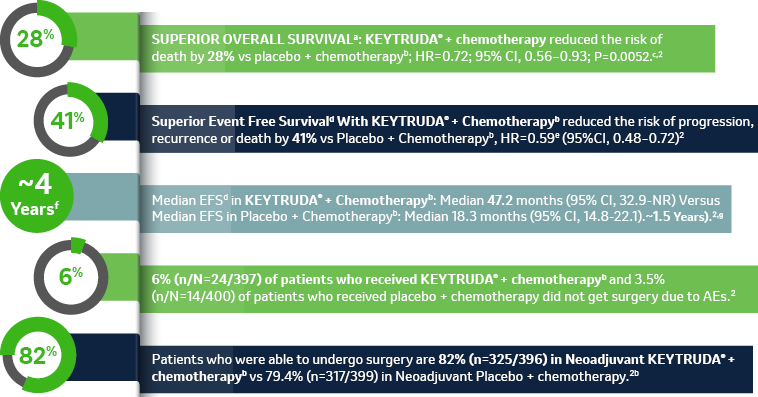
aOS defined as time from randomization to death from any cause2, bChemotherapy: Cisplatin 75 mg/m2 IV on Day 1 + (pemetrexed 500 mg/m2 IV on day 1 or gemcitabine 1,000 mg/m2 IV on days 1 and 8) of each 21-day cycle for up to 4 cycles, cThe multiplicity-adjusted superiority threshold for overall survival at the second interim analysis was one-sided p=0.0054.2
dEFS is defined as the time from randomization to the first occurrence of local progression that precluded the planned surgery, unresectable tumor at the time of surgery, progression or recurrence according to RECIST v1.1, or death from any cause,
eBased on Cox regression model with treatment as a covariate stratified by stage, tumor PD-L1 expression, histology, and geographic region2,
f3.933 years (47.2 months),
g1.525 years (18.3 months).
CI = confidence interval; HR = hazard ratio; NR = not reached; NSCLC = non–small cell lung carcinoma; OS = overall survival; PD-L1 = programmed death ligand 1, AE = adverse event.
References:
- KEYTRUDA® Prescribing Information.
- Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Chen KN, Dooms C, Majem M, Eigendorff E, Martinengo GL, Bylicki O, Rodríguez-Abreu D, Chaft JE, Novello S, Yang J, Arunachalam A, Keller SM, Samkari A, Gao S; KEYNOTE-671 Investigators. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024 Sep 28;404(10459):1240-1252. doi: 10.1016/S0140-6736(24)01756-2. Epub 2024 Sep 14. PMID: 39288781.
JO-KEY-00347. Expiry Date: 30.October.2025